

Miracle fertilizer
Fertilizer isn’t literally a miracle, but it has been confused with one. In recent years Evangelical Christians have been converting Guatemalans away from Catholicism. The results, some Guatemalans say, are fantastic. Not only has drunkenness fallen, but people have been raised from dead. The fields even produce more food!
Did these religious conversions really reduce drunkenness? Perhaps. Did they raise people from the dead? Of course not. Did God, pleased with the conversions, bless the field so that it was more fertile? Probably not, considering that these conversions occurred at the same time chemical fertilizers were starting to became available to the region.
[A Guatemalan citizen states] “God started moving in the whole community, through different miracles that happened—people were getting saved, even I heard miracles of people being raised from the dead, [curing alcoholism], families being restored, you didn’t see all the drunks on the street anymore. It was a complete change.” And according to Evangelical lore, not only did the character of the people ... change, the God blessed and healed the land so they could grow more vegetables, and big vegetables, and the records show the vegetables got bigger.”
—Cole, Sean. March 19, 2013. The Story. “Spiritual Warfare: Evangelical Protestants Convert Catholics.” American Public Media.
“... a leading Guatemalan economist, published an article about [a Guatemalan region] in which he points out that the mass conversion of Evangelism occurred around the same time as chemical fertilizers, new seeds, and new crops were being introduced to Guatemala.”
—Cole, Sean. March 19, 2013. The Story. “Spiritual Warfare: Evangelical Protestants Convert Catholics.” American Public Media.
This story testifies to the efficacy of chemical fertilizers. They increase a field’s fertility so much that it is mistaken for a divine blessing! Divine or not, these fertilizers are indeed a blessing. Without modern nitrogen fertilizers we would be unable to feed 35% of today’s population. These “modern” fertilizers we spoke of are chemical fertilizers. Nitrogen fertilizer is made in large factories by using advanced chemistry and fossil fuels to extract nitrogen from the atmosphere and convert it to a form plants can consume. So successful are these factories that half of all the nitrogen in an American’s body can be traced back to these fertilizer factories. Other fertilizers like phosphorus and potassium are acquired by mining deep into the earth.
“Song Linyuan, an elderly but spry farmer in a village northwest of Nanjing, recalls how he once kept his 1.3 acres of cropland as fertile as possible, composting household waste and spreading manure from his pigs and chickens. In all, his efforts added perhaps a hundred pounds of nitrogen per acre of land each year. He harvested 2,600 to 3,300 pounds of rice per acre.
That’s a respectable harvest, a better yield than in many parts of the world. But now he gets more than twice that: 7,200 pounds per acre. It’s a harvest many farmers can only dream of.
The difference? ‘Better fertilizer,’ he says. We’re sitting in a shop surrounded by farmers. Song’s answer provokes a loud discussion. Some agree that fertilizer was key; others say better seeds were more important. In reality the two technologies are intertwined. The high-yielding varieties of rice and wheat that breeders created in the 1950s and 1960s could reach their full potential only if they got more nitrogen.”
—Charles, Dan. May 2013. “The Curse of Fertilizer.” National Geographic.
N, P, and K
Plants need a variety of nutrients, but the main nutrient limiting the productivity of a soil is nitrogen (N), phosphorus (P), and potassium (K). This is why most fertilizers have only N, P, and K. Can a farmer apply only these three fertilizers to a field and maintain fertility forever? No, as plants have other nutrient needs. However, because these other nutrients are usually found in ample amounts in the soil, farmers can produce high yields using only N, P, and K fertilizers for decades—even centuries.
Fertilizer, lesser-nutrients, and the nutrient content of food
Ancient China once found itself a vassal of the Mongolians, paying tribute because it could not defeat Mongolian horsemen in battle. The Mongols had stronger, faster horses, while Chinese horses were much weaker. Two or more could be used to pull a chariot, but they could not be ridden by a mounted warrior. Why? According to historians, this is because the Chinese soils were deficient in the lesser-nutrient selenium, a nutrient needed for strong bones, while the Mongolian soils contained an abundance. The Mongols were thus superior in battle because their soils were superior in nutrients.(H1) Sometimes, it is the soil that makes the nation. For the Mongolians, their soil was their secret weapon.
Most of the time the word “fertilizer” refers to plant available nitrogen (N), phosphorus (P), and potassium (K), yet there are many other nutrients essential for plants and animals. There is carbon, oxygen, and hydrogen that plants acquire from the air or water. Other elements, most of which are acquired from the soil, are ...
- calcium
- magnesium
- sulfur
- boron
- chlorine
- copper
- iron
- manganese
- molybdenum
- zinc(T2)
These nutrients other than N, P, and K I refer to throughout this lecture as lesser-nutrients. This is because they are needed in lower amounts relative to N, P, K, carbon, oxygen, and hydrogen.
There are some lesser-nutrients barely needed by plants, but are necessary for animal growth. Examples are selenium and iodine. Plants generally have little need for them, but animals do, and plants give us these nutrients by embedding it into the vegetation that animals eat. Plants can often be healthy and provide large yields, even if they grow in selenium- or iodine-deficient soils.
Consider iodine. All humans need it, but in low amounts. Mothers with excessively low iodine levels in their womb have children who score worse on literary tests, so just because you don’t hear much about iodine doesn’t mean it isn’t a problem.(R1) Look at the label of the salt you buy and you will probably see that it also contains iodine (unless it is Kosher or pure Sea Salt). Since we all use small amounts of salt each day, the best way to ensure we consume small amounts of iodine is to add it to salt. Some individuals do not like the taste of iodine in salt and choose Kosher salt instead. The Nutrition Diva Monica Reinagel says this is okay, as, “Vegetables can be a source of iodine, depending on the iodine content of the soil in which they’re grown.” Iodine may sound like a trivial concern, but recent research suggests that even with iodized salt pregnant women may not be receiving enough.(C1,G1,R2)
If a soil is being depleted of important lesser-nutrients, we could suffer the consequence. Many people in developing countries where iodine is not added to salt, and whose soils are lacking in iodine, suffer the consequences in the form of malnutrition from iodine deficiency. This is important, because the health of the plant doesn’t tell us everything about its nutrient composition, which means a farmer can harvest a healthy crop without realizing it is deficient in iodine. Some minerals important to human health are not actually used by the plant, so the plants could be remain healthy even if its nutrient content for humans is in decline.(E1)
Although they don’t get much attention in the U.S., lesser-nutrients are important. We don’t hear much about them because they are usually so abundant in the soil that they pose no problem. One day, though, they might, so we should study them now instead of waiting until their absence causes a health problem.
Critics of chemical fertilizers rightly point out that N, P, and K aren’t everything a plant needs, and farming without returning many of these lesser-nutrients to the field means you are basically mining the soil. How long can we continue to mine the soil? Some estimates for some specific locations suggests there are enough lesser-nutrients for hundreds, sometimes thousands, of years.(W3)
Sometimes a lesser-nutrient problem exists even though it is prevalent in the soil. This is because not all nutrients are available to plants. North Dakota soils have large amounts of iron but much of it is unavailable because the soil’s pH is too high. The solution is not to apply more iron but to lower the soil’s pH, or to apply a chelate, which helps transport iron from the soil onto the surface of plant roots.(N1)
We have an answer for what will happen when a soil becomes deprived of lesser-nutrients, because in a few areas it has already happened. In the last twenty years some wheat farmers in the state of Washington noticed that applying more nitrogen did not increase yields, which suggested the wheat’s growth was limited by the absence of two other nutrients: chlorine and sulfur. How did Washington farmers respond? Fertilizer companies developed a market to profit off this need, and so farmers today easily acquire inexpensive chloride and sulfur, restoring high yields.(S1) When some other lesser-nutrient becomes scarce in the soil, fertilizer companies and farmers will respond in the same way. In fact, in areas like Oklahoma where lesser-nutrients are not a problem, there are fertilizer salespeople trying to sell fertilizer supplements. When agronomists test the performance of these supplements, they usually find they have no impact on yield.(Z1) Now, just think how many salespeople would be marketing copper, iron, and boron, and zinc in Oklahoma if it was actually needed!
Fertilizer and the nutrient content of food
Some people have observed that the nutrient content of many foods has fallen over time, and they attribute this to a lack of lesser-nutrients in the soil. Is our reliance on chemical fertilizers causing our food to be less nutritious?
Research suggests the blame mostly belongs to new crop varieties. With the rise of chemical fertilizer, also came higher yielding crop varieties. These more efficient varieties of grains, fruits, and vegetables are subject to the genetic dilution effect, a concept describing the tradeoff between yield and nutrition. These improved varieties of plants achieve a higher yield in two ways (1) by taking more nutrients from the earth and/or (2) by packing less nutrients per pound of food. Most studies find that the new crop varieties—not a reduction of nutrients in the soil—are probably the major source of nutrient loss in foods today.(D1,D2)
This was illustrated nicely on the Broadbalk fields in England, where experimental plots have been maintained since 1843. Between 1843 and the 1960s, the concentration of lesser-nutrients in the wheat harvested remained steady, but then began falling, giving the impression that the soil was running out of zinc, iron, copper, and magnesium. However, the actual amount of [plant available] lesser-nutrients in the soil had remained steady or increased, leading researchers to conclude it was the choice of wheat varieties planted that reduced the nutrient content of the wheat, not soil deficiencies.(F1)
Of course, the nutrient content of food is less important than access to total nutrients, and over the last century the U.S. has increased its per capita production of almost every nutrient (and for the nutrients that are less available today, the decrease is small).
Figure 1—Nutrient content of food (per capita) today compared to a century prior

Greater nutrient availability doesn’t necessarily mean greater nutrient consumption, if patterns of food waste are changing at the same time more nutrients are produced. Studies of nutrient consumption in the UK find that the per-person intake of some lesser-nutrients like magnesium, iron, zinc, and copper has fallen over time, and in some cases is insufficient for a person’s daily needs. While there are nutrient deficiencies in the U.S. they have not changed much since 1999.(F1) The purpose of the above figure is not to argue that there are no lesser-nutrient deficiencies in the U.S., but that the persistent use of chemical fertilizers does not seem to pose a nutrient problem.
Another way to inquire whether chemical fertilizers affect the nutrient content of food, is to compare non-organic food to organic food. Most of the time, non-organic food is raised using chemical fertilizers, whereas organic food must use other sources (e.g., manure, compost, crop rotations). Many scientific comparisons have been made, but the overall results suggest organic food is equivalent to conventional food in terms of nutrition.(B1,D3,W1) When grocery stores in the United Kingdom tried to market organic food as being more nutritious, it was ordered by the government to stop, because it was considered false advertising. The stores could not refute the government’s accusation, so they ceased advertising organic as nutritionally superior.(I1) Some more recent research finds that organic food may have more antioxidants(C3), so perhaps future studies will deem organic food as more nutritious. Organic food does have less pesticide residues, but we defer this issue to the lecture on pesticides.
More than just dirt
If you want to frustrate a plant scientist, just refer to soil as “dirt.” This drives them crazy, and for a good reason. Thus far I have been discussing crop production as if the soil was a mere container to hold nutrients like nitrogen—a mere dinner plate on which food is placed. A dinner plate is a very poor metaphor, because the soil is not a substance, but an ecosystem.
Just as health experts are learning all the ways our bodies are influenced by the micro-organisms in our intestine, plant scientists are discovering myriad ways that organisms within the soil affect plant growth. Plants often grow best when the soil is [almost] literally alive, containing a gallery of bacteria, fungi, worms, insects, and other critters. In just a teaspoon of soil there can be between 100 million and 1 billion bacteria!(D5) Tunnels dug by earthworms help aerate the soil. Some insects prey upon crop pests, thereby preventing crop damage. Some fungi settle on plant roots, helping plants communicate with one another by warning other plants of an approaching pest.
(If the idea of plants communicating surprises you, you have underestimated the complexity of plants, which can not only communicate, but have 15-20 different senses. Compare that to our measly five senses of smell, taste, sight, touch, and sound.(P1))
There are some microbes that assist plants in acquiring nutrients from the soil. A bacterium named Paenibacillus can prevent tomatoes from being contaminated with salmonella. Other micro-organisms help assimilate carbon into the soil, thus reducing greenhouse gases concentrations in the atmosphere. There are bacteria living on the roots of legumes which snatch nitrogen from the air and convert it a plant-accessible for of nitrogen.
“‘Even though this looks static, like there’s nothing going on out there, beneath the surface there is something going on. All winter long, I’ve got worms and microbes preparing for the planting season.’ He recalls seeing one estimate that untilled fields contain 30 million earthworms per acre, versus 3 million in the plowed soil.”
—Johnson, Nathaniel [reporter] and David Ausberger [farmer and interviewee]. March 13, 2014. “Un till: An Iowa farmer finds that
less (plow) is more (profit)” Grist. Accessed March 14, 2014 at http://grist.org/food/un-till-an-iowa-farmer-finds-that-less-plow-is-more-profit/?utm_source=syndication&utm_medium=rss&utm_campaign=feed.
We should recognize that microbes are not always beneficial for crops. Sometimes they help the pests that damage crops, like the bacteria living inside the Colorado potato beetle. Normally, when the potato plant is being eaten, it recognizes the presence of a herbivore and erects its herbivore-relevant defences. However, the bacteria in the beetle tricks the plant into thinking that the beetle is a microbe, and so the plant erects the wrong type of defenses—and the beetle thrives.(S3) The job of agricultural scientists is to encourage the good microbs and discourage the bad.
Figure 2—The Soil Food Web

Many of these beneficial organism populations are reduced by chemical fertilizers, although the negative effects from such seem to be small. In cases where the effects are large, one can either reduce the use of chemical fertilizers or replace it with manure or compost. There is also the option of retaining the use of chemical fertilizers, but finding ways to compensate for the loss of organisms. For instance, the aeration of the soil normally performed by earthworms can be done with mechanical aerators instead. If fertilizers are killing large numbers of microbes, there are companies like Terra-One that sell fertilizer supplements that replace these populations (gardeners have been purchasing microbes for decades).
Healthy soils are high in organic matter, as it helps prevent erosion and retain moisture. Soils low in organic content can be improved by applications of manure or compost, or by the adoption of no-till methods, where the ground is never plowed and the roots formed by plants remain in the soil, undisturbed. Some farmers have actively sought to raise the organic matter in the soil, and have placed a monetary value on the organic matter at $3,775 per acre.
Just as some microbial colonies must be allowed to thrive naturally to preserve soil health, other microbes can be introduced into new areas to aid a degrading soil. Cropland under irrigation tends to suffer from salinity, as ground water contains levels of salt not present in rain. This detracts from crop production, but some areas have no choice but to use irrigation. One specific problem with high salt levels is that it prevents the formation of bacteria on legume plant roots, bacteria which can convert atmospheric nitrogen into a plant-accessible form. Researchers are optimistic that this problem can be mitigated by introducing bacteria from the Pseudomonas family into the soil.(A2,C2,N3,E1,W2)
Soil erosion
Soil may not be dirt, but a field that loses most of its topsoil essentially becomes dirt. Not only do the nutrients wash away with the topsoil, but the ecosystem goes with it. The dirt underneath topsoil has a different texture, and is usually less suitable for crop production. The point is that the topsoil is a resource, and we must conserve it as best we can. However, it is a lamentable fact that it is nearly impossible to raise crops without some soil erosion. Erosion is a natural part of life, even in the absence of humans, but crops are raised by plunging plows, cultivators, and/or planters into the ground, loosening the soil and leave it exposed, making it that much easier for rainstorms and winds to take it away.
Erosion can be reduced to low levels by adoption of no-till planting methods where seeds are planted directly into an unplowed soil and weeds are managed using pesticides. The creation of transgenic soybeans, for instance, has made it much easier for farmers to control weeds with the herbicide Round Up, thus encouraging farmers to abandon the plow and adopt no-till methods which can reduce erosion by as much as 90%.(N2) Of course, this means greater pesticide use and reliance on transgenic crops, which some oppose. The point is that the soil is a valuable resource that must be protected, but such protection always comes at a cost.
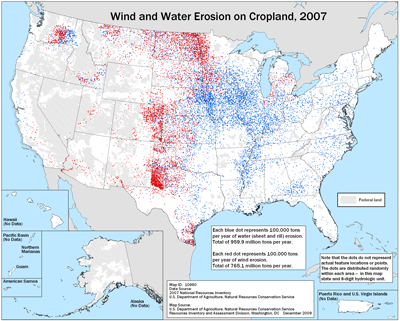
The figure below shows that erosion is indeed a national problem, but we are also doing better, as less soil was lost in 2007 compared to 1982. A major reason is indeed the adoption of transgenic crops, the pesticide Round Up, and various no-till production methods. When we debate whether we want to use synthetic pesticides and transgenic crops, we are also debating how much soil erosion we can tolerate. It is also the result of a change in culture. As technology makes no-till practices easier to implement farmers and agricultural scientists start thinking more about soil erosion, and the more we think about it, the higher we value that precious ecosystem that gives us life.(H4)
Figure 3—Soil erosion in 1982
(Blue and red dots represent 100,000 tons of annaul erosion due to water and wind erosion, respectively)
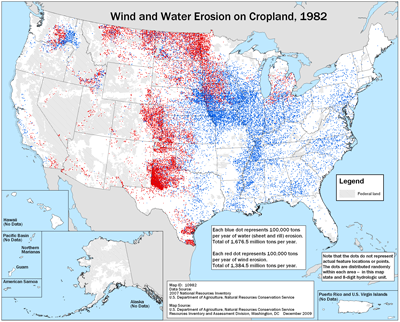
Figure 4—Soil erosion in 2007
(Blue and red dots represent 100,000 tons of annaul erosion due to water and wind erosion, respectively)
Figures
(1) Original figure from source (N3).
(2) By USDA [Public domain], via Wikimedia Commons.
(3) and (4) National Resource Conservation Service. Soil Biology Primer [website]. United States Department of Agriculture. Accessed May 10, 2014 at http://www.nrcs.usda.gov/wps/portal/nrcs/detail/national/technical/nra/dma/?cid=stelprdb1041887.